FeNO home monitoring: Clinical benefits and practicality
FeNO is a recognized biomarker for Type 2 inflammation in asthma. While doctor's measurements are in guidelines, the additional benefits of home monitoring have been less clear. The FeNO@home study published in 2024 sheds new light on this crucial aspect of asthma management.

Traditional asthma management
Many patients do not reach their full therapeutic potential

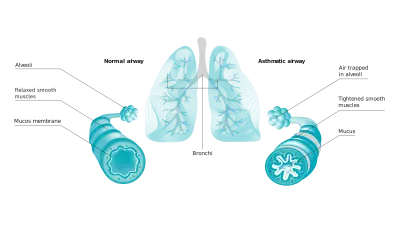
Current asthma treatment with inhaled drugs and biologics aims at achieving symptomatic control and preventing future risk. This approach has recently been extended by adding long-term remission as a realistic goal of asthma therapy.1 But the current reality differs from the theory and guidelines: Around 40% of asthma patients are not adequately controlled.2 Why? A key point is that the traditional concept of asthma control does not involve any biomarker, even though airway inflammation plays a crucial role in the chronic disease. More specifically: Most asthma cases arise from Type 2 inflammation – an overreaction of the immune system.
Fractional exhaled Nitric Oxide (FeNO) is a well-established marker of Type 2 inflammation. Elevated FeNO supports an asthma diagnosis in clinical routine.1 Asthmatics with increased FeNO levels are at higher risk of exacerbations and progressive lung function decline.3 FeNO levels above normal are linked to greater treatment benefits with anti-inflammatory drugs including certain biologics.4 This is why more and more leading experts regard FeNO as an indispensable part of phenotyping and precision medicine.1
The FeNO@home study
Additional benefits of FeNO home monitoring

Evidence in scientific literature relates primarily to sporadic FeNO measurement in the doctor's office. What has not yet been investigated to the same extent is the additional benefit of FeNO monitoring by patients at home. In this regard, the FeNO@home study provides new, groundbreaking insights.5 It was conducted as a multicenter study in Germany and Italy. Asthma patients of varying severity were enrolled. Furthermore, the study tested how the Vivatmo me patient device from Bosch performs in everyday use. Starting with the first presentations at the ERS Congress 2024, the results will now gradually be published in renowned journals.
FeNO@home: Clinical benefits at a glance
FeNO home monitoring provides significant additional benefits over single measurements by doctors.5,7,9
Pattern recognition
Doctors can refine diagnosis and therapy by examining a patient's long-term inflammatory profile and app-recorded context data.
Higher Type 2 sensitivity
FeNO home monitoring sensitivity for Type 2 asthma is up to 5.3 times higher than sporadic doctor's measurements.
Optimized medication
FeNO home monitoring allows for more precise selection of biologics and tracking of drug response in near real-time.
Paving the way to true asthma remission
Explore study results in a nutshell – FeNO@home infographics5,7,8,9
Going beyond traditional concepts
Long-term inflammation course provides a more holistic picture
A major challenge in clinical asthma management is the lack of robust, objective, longitudinal data on the individual disease course. Cross-sectional data gathered at individual time points during practice visits may suffer from the inherent variability of asthma and therefore are not fully representative. As a consequence, diagnosis and treatment may not be appropriately adapted to the patient’s individual inflammatory profile. Thus, patients may receive inappropriate medication, such as inhaled corticosteroids (ICS) in unsuitable doses, or they may seem ineligible for certain biologics.
FeNO home monitoring helps to solve this problem: Compared to sporadic FeNO tests in the doctor’s office, continuous home monitoring provides additional advantages, as the course of inflammation creates a more holistic clinical picture. FeNO@home5,7 has revealed recurring patterns of inflammation – characterized by FeNO variability, median, and extremes. Ideally, FeNO is recorded together with other context data to enable optimal analyses of the patterns and cause-effect relationships – for example in conjunction with symptoms, lung function parameters, infections, medication, or allergen exposure. Patients’ individual FeNO profiles can be used for trigger identification and clinical decision-making to optimize personalized asthma management. Evidence suggests that a treat-to-target approach adding FeNO to the management strategy can improve asthma control.6

How does FeNO measurement at home work?

Loading the video requires your consent. If you agree by clicking on the Play icon, the video will load and data will be transmitted to Google as well as information will be accessed and stored by Google on your device. Google may be able to link these data or information with existing data.
This video explains how easy it is for patients to perform FeNO measurement at home with Vivatmo me by Bosch.
Quick and reliable home testing with Vivatmo me
The world’s first and only patient device for FeNO home monitoring

There is only one system in the world that is approved for FeNO monitoring at home: Bosch Vivatmo me accompanied by the Vivatmo app. The patient device enables quick, easy, and non-invasive FeNO testing anywhere and at any time. FeNO values are transferred to the app via Bluetooth. In their digital asthma diary, patients can also document other relevant data such as symptoms, pollen count, or medication. The app’s monthly reports can be shared with doctors. In the FeNO@home study, doctors and patients confirmed the excellent usability, high adherence, and therapeutic benefits of Vivatmo me.7
Ideal duo: Office and home measurement


References
1 Lommatzsch et al. Pneumologie 2023;77:461-543
2 Global Asthma Report 2022: 30
3 van Veen et al. Eur Respir J 2008; 32: 344-49
4 Price et al. Lancet Respir Med 2018;6:29-39; Lee et al. Lancet Respir Med 2021;9:69-84; Pavord et al. JACI Pract 2023;11:1213-20
5 Beeh et al. 2022: Options to control asthma by means of a newly developed FeNO device for use at home [FeNO@home study].German Clinical Trials Register: DRKS00029118
6 Powell et al. Lancet 2011; 378:983-990
7 Beeh et al.: Benefit of daily FeNO measurement in asthmatics over 12 weeks. Poster presented at ERS 2024
8 Louis et al. Eur Respir J 2022 60: 2101585
9 Individual case report (publication in preparation) from Beeh et al. 2022: Options to control asthma by means of a newly developedFeNO device for use at home [FeNO@home study]. German Clinical Trials Register: DRKS00029118
This website contains general product information about the Vivatmo system from Bosch. Not all products and their functions mentioned here are approved in every regional market. For a detailed description of the products and features as well as information on intended and safe use, please refer to your locally authorized Bosch distribution partner and the instructions for use that are valid for your country.